Thank you for recognition | JUVE Best commercial law firms 2021/2022
We are thankful to our team, our partners, and clients that we can jointly improve medical care by innovative legal and strategy consulting in healthcare and life sciences. It is great to see that our work and effort on making an impact has again received a high recognition. In this year’s JUVE Directory Dierks+Company has been recognized as one of the best commercial law firms for pharmaceutical and medical device law.
The new JUVE Directory of Commercial Law Firms 2021/2022 was published today, 29th of October 2021. It gives a detailed overview of the scope of Dierks+Company, its strengths and some quotes and recommendations from clients.
About JUVE
JUVE, a Cologne based press publisher, reports in their journals and manuals, as well as on its websites, about the business law market in Germany and Austria, with the aim of offering orientation in specialized economic sectors.
Their publications address lawyers, patent attorneys and tax advisors in law firms, consulting firms, companies, the judiciary, and administration, as well law students, trainee lawyers, doctoral candidates – the lawyers and tax advisors of tomorrow.
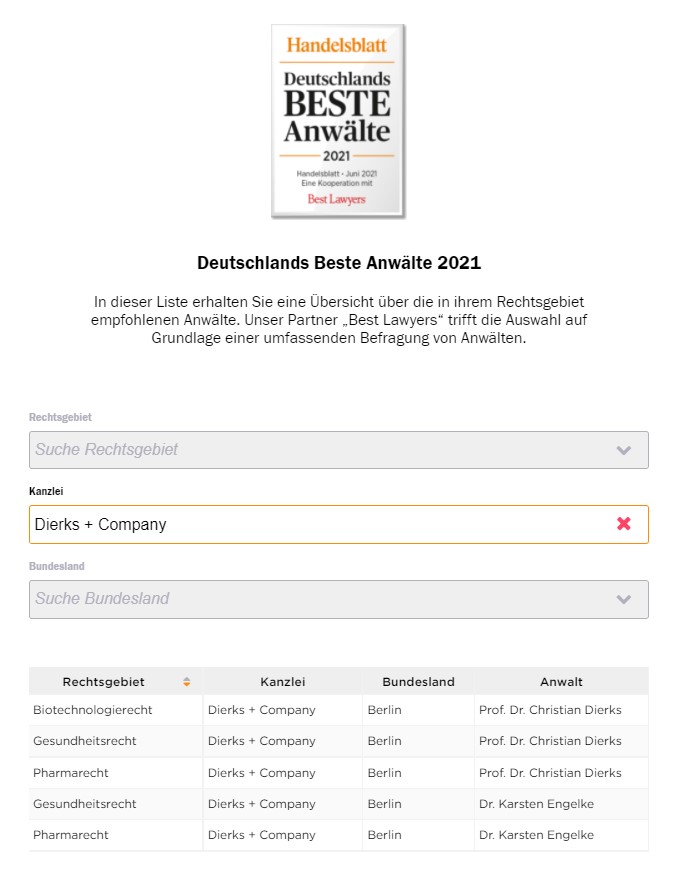
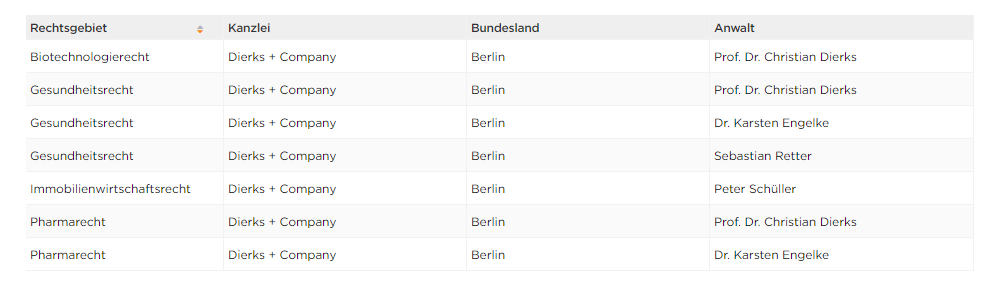
Best Lawyers has named Christian Dierks as Lawyer of the Year in Biotechnology and Life Sciences Practice and Best Lawyer in Healthcare and Pharmaceutical Law. Karsten Engelke is also among this year's Best Lawyers in Healthcare and Pharmaceutical Law. The overview of the US publisher Best Lawyers was published exclusively in Handelsblatt on June 25. Christian Dierks has been listed by Best Lawyers for legal advice in the areas of biotechnology, healthcare and pharmaceuticals since 2009.
"Our thanks go to our colleagues in the legal market whose recommendations have earned us this honor. We are pleased to be able to work with the other attorneys listed here to help improve medical care and drug development and approval through innovative legal advice in the health care industry. It is great to see that this work is receiving such high recognition," emphasizes Christian Dierks.
The Best Lawyers rankings are determined in an elaborate peer-to-peer process by Germany's most renowned legal advisors. Leading lawyers are asked in this process which colleagues they recommend - recommendations for their own law firm are excluded. The result is a comprehensive overview of the "Law Firms of the Year 2022" and the "Best Lawyers of the Year 2022," which are published exclusively in Handelsblatt and on Best Lawyers online.
"We have very ambitious growth targets at Dierks+Company. The Best Lawyers award helps us to attract the best minds for our innovation consultancy for healthcare and life sciences," emphasizes Head of People + Culture Volker Westedt. "In our innovation consulting, we combine legal and medical expertise to create innovative solutions in research and care. To do this, we need the right thought leaders and strategists who not only want to accompany the transformation of healthcare, but also shape it. The fact that lawyers all over Germany are voting us Law Firm of the Year gives us just the right tailwind to do so."
We are very happy to announce that Dierks+Company has been recognized by Best Lawyers in 2020.
Best Lawyers is one of the leading international recommendation portals for lawyers. Only those who have been rest Lawyers is one of the leading international recommendation portals for lawyers. Only those who have been recommended by other professional colleagues listed in a complex procedure are included in the rankings. This ensures a high level of reliability of the recommendation lists. In Germany, the rankings have been published exclusively by the Handelsblatt for many years.
The results are available, where 4 lawyers from our law firm are among the "Best Lawyers" in Germany. The recommendations are spread across these specialist areas: Biotechnology law, health law, pharmaceutical law and real estate law.
The following lawyers of Dierks+Company were included in the circle of "Best Lawyers":
We are very pleased to announce that Dierks+Company has been recognized by the business magazine “brand eins” as one of the best commercial law firms.
"Brand eins" has evaluated more than 2,600 expert judgments: 398 business law firms have made it onto the leaderboards, including Dierks+Company in the Healthcare and Pharmacy category.

The digital application for the registration of corona patients was not included in the legislative package for the protection of the population after all.
Would it be permissible under data protection law?
Christian Dierks answers the question. You can read the article here, on e-health.com.de. (German)
The legal framework currently in place in the Federal Republic of Germany for the processing of personal data for research purposes is characterized by a network of standards comprising the DS-GVO, the Federal Data Protection Act, the State Data Protection Acts and the State Hospital Acts with independent regulations on research with patient data. In the normal case of a research association with hospitals under different sponsorship across state borders, the legislative patchwork leads to a variety of regulations that is difficult to overlook. This results in considerable legal uncertainties and disadvantages for the attractiveness of Germany as a research location.
Against the background of this problem, the following legal opinion from Dierks+Company discusses potential options for action to simplify the use of health data for research purposes, some of which have already been implemented with the recent law on the protection of the population (“Bevölkerungsschutzgesetz”).
You can read more about the legal opinion in German on our Publications page.
The legal opinion was prepared by Prof. Dr. Dr. Christian Dierks, with the collaboration of Dr. Philipp Kircher, Charlotte Husemann, Dr. Karsten Engelke, Julia Pirk and Dr. Martin Haase, and is publicly available on the official website of the Federal Ministry of Health.
Localization systems, emergency call or fall detection systems, but also shutdown systems for household appliances or digital aids to remind people to take food and drink are Ambient Assisted Living technologies. Ambient Assisted Living technologies, in short AAL, have a nursing benefit by enabling people in need of care to lead independent lives and at the same time relieving the burden on nursing staff.
The expert report shows how the legal regulations in the German Social Security Code (SGB V and XI) can be adapted to integrate digital assistance systems as care aids into standard care.
During the last few weeks, the report got very popular in online media (and also in offline media).
Here are some of them:
- www.vzbv.de/pressemitteilung/digitale-pflegehelfer-kassen-sollen-kosten-erstatten
- www.aerzteblatt.de/nachrichten/109326/Rechtsgutachten-empfiehlt-digitale-Pflegehelfer-als-Kassenleistung
- www.zm-online.de/news/politik/kassen-sollen-digitale-pflegehelfer-erstatten/
- www.healthcaremarketing.eu/publicaffairs/detail.php?rubric=Politik&nr=67853&PHPSESSID=ia12gfck7319ch2gr9dfe20485
- www.evangelisch.de/inhalte/165872/12-02-2020/digitale-helfer-der-pflege-kaum-erstattungsfaehig
- www.mednic.de/verbraucherzentralen-fordern-kostenerstattung-fuer-digitale-assistenten/13481
- www.krankenkassen-direkt.de/news/mitteilung/vzbv-Digitale-Pflegehelfer-Kassen-sollen-Kosten-erstatten-2444784.html
- www.verbaende.com/news.php/Digitale-Pflegehelfer-Kassen-sollen-Kosten-erstatten-Rechtsgutachten-empfiehlt-gesetzliche-Anpassungen?m=133102
- www.aerztezeitung.de/Politik/Mit-virtuell-betreutem-Wohnen-die-Pflege-entlasten-406684.html
- www.schwarzwaelder-bote.de/inhalt.sturzsensor-und-herdabschaltung-kassen-sollen-pflegehilfen-zahlen.a4017c12-6f68-46a6-81da-f11ed287c5ff.html
- www.e-health-com.de/details-news/pflegekassen-sollen-fuer-pflege-aal-zahlen/
The report was prepared by Christian Dierks, Sebastian Retter, and Julia Pirk, on behalf of the Federation of German Consumer Organisations.
It is available to read and download here (German).
This volume offers an overview of the current legal framework to researchers and all other interested parties who wish to use social and health data. The work also enables the healthcare industry and government institutions to develop solutions and products that improve the quality of care. Thus, this publication gives actors in the health care system an important orientation for the legally compliant use of social and health data. In the first part, the social-legal framework for the use of social data for research is presented. The second part provides an overview of the handling of research data after application of the DSGVO (General Data Protection Regulation) and corresponding national adaptations of the legal framework.
You can read more about the book in German on our Publications page.
The book is available for purchase here.
In the recently published blog on the e-health-com online portal, Christian Dierks responds to the question: Which legal requirements should actually be observed for research with patient data in cross-border clinical research projects?
The blog post with the complete answer is available here (German).
The German data protection patchwork creates unnecessary barriers for health research. Germany therefore has a disadvantage compared to other EU countries that we can and should overcome. - Guest article form Christian Dierks was published in the Handelsblatt Inside Digital Health newsletter.
The article is available here (German).




